Hippocampus
-
Нейронное декодирование раскрывает секреты навигации

Анализируя закономерности срабатывания нейронов направления головы и «клеток места», теперь можно точно предсказать местоположение и ориентацию мыши, проливая свет на сложные функции мозга, участвующие в навигации.
https://neurosciencenews.com/neural-navigation-25655/
https://www.cell.com/biophysj/fulltext/S0006-3495(24)00041-9
-
Delayed TBI-Induced Neuronal Death in the Ipsilateral Hippocampus and Behavioral Deficits in Rats: Influence of Corticosterone-Dependent Survivorship Bias?
Acute and chronic corticosterone (CS) elevations after traumatic brain injury (TBI) may be involved in distant hippocampal damage and the development of late posttraumatic behavioral pathology. CS-dependent behavioral and morphological changes were studied 3 months after TBI induced by lateral fluid percussion in 51 male Sprague–Dawley rats. CS was measured in the background 3 and 7 days and 1, 2 and 3 months after TBI. Tests including open field, elevated plus maze, object location, new object recognition tests (NORT) and Barnes maze with reversal learning were used to assess behavioral changes in acute and late TBI periods. The elevation of CS on day 3 after TBI was accompanied by early CS-dependent objective memory impairments detected in NORT. Blood CS levels > 860 nmol/L predicted delayed mortality with an accuracy of 0.947. Ipsilateral neuronal loss in the hippocampal dentate gyrus, microgliosis in the contralateral dentate gyrus and bilateral thinning of hippocampal cell layers as well as delayed spatial memory deficits in the Barnes maze were revealed 3 months after TBI. Because only animals with moderate but not severe posttraumatic CS elevation survived, we suggest that moderate late posttraumatic morphological and behavioral deficits may be at least partially masked by CS-dependent survivorship bias.
-
Detailed Analysis of Dorsal-Ventral Gradients of Gene Expression in the Hippocampus of Adult Rats
We performed RNA sequencing of the dorsal and ventral parts of the hippocampus and compared it with previously published data to determine the differences in the dorsoventral gradients of gene expression that may result from biological or technical variability. Our data suggest that the dorsal and ventral parts of the hippocampus differ in the expression of genes related to signaling pathways mediated by classical neurotransmitters (glutamate, GABA, monoamines, etc.) as well as peptide and Wnt ligands. These hippocampal parts also diverge in the expression of axon-guiding molecules (both receptors and ligands) and splice isoforms of genes associated with intercellular signaling and cell adhesion. Furthermore, analysis of differential expressions of genes specific for astrocytes, microglia, oligodendrocytes, and vascular cells suggests that non-neuronal cells may also differ in the characteristics between hippocampal parts. Analysis of expression of transposable elements showed that depletion of ribosomal RNA strongly increased the representation of transposable elements in the RNA libraries and helped to detect a weak predominance of expression of these elements in the ventral hippocampus. Our data revealed new molecular dimensions of functional differences between the dorsal and ventral hippocampus and points to possible cascades that may be involved in the longitudinal organization of the hippocampus.
-
Early Life Events and Maturation of the Dentate Gyrus: Implications for Neurons and Glial Cells
The dentate gyrus (DG), an important part of the hippocampus, plays a significant role in learning, memory, and emotional behavior. Factors potentially influencing normal development of neurons and glial cells in the DG during its maturation can exert long-lasting effects on brain functions. Early life stress may modify maturation of the DG and induce lifelong alterations in its structure and functioning, underlying brain pathologies in adults. In this paper, maturation of neurons and glial cells (microglia and astrocytes) and the effects of early life events on maturation processes in the DG have been comprehensively reviewed. Early postnatal interventions affecting the DG eventually result in an altered number of granule neurons in the DG, ectopic location of neurons and changes in adult neurogenesis. Adverse events in early life provoke proinflammatory changes in hippocampal glia at cellular and molecular levels immediately after stress exposure. Later, the cellular changes may disappear, though alterations in gene expression pattern persist. Additional stressful events later in life contribute to manifestation of glial changes and behavioral deficits. Alterations in the maturation of neuronal and glial cells induced by early life stress are interdependent and influence the development of neural nets, thus predisposing the brain to the development of cognitive and psychiatric disorders.
-
Glucocorticoid‐mediated mechanisms of hippocampal damage: Contribution of subgranular neurogenesis
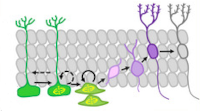
Our employees from the Laboratory of Functional Biochemistry of Nervous System presented a comprehensive overview of the interplay between glucocorticoids (GCs) and adult hippocampal neurogenesis (AHN), particularly, in the context of a diseased brain. The effectors of GCs in the dentate gyrus neurogenic niche of the hippocampal are reviewed, and the consequences of the GC signaling on the generation and integration of new neurons are discussed. Recent findings demonstrating how GC signaling mediates impairments of the AHN in various brain pathologies are overviewed. GC‐mediated effects on the generation and integration of adult‐born neurons in the hippocampal dentate gyrus depend on the nature, severity, and duration of the acting stress factor. GCs realize their effects on the AHN primarily via specific glucocorticoid and mineralocorticoid receptors. Disruption of the reciprocal regulation between the hypothalamic–pituitary–adrenal (HPA) axis and the generation of the adult‐born granular neurons is currently considered to be a key mechanism implicating the AHN into the pathogenesis of numerous brain diseases, including those without a direct hippocampal damage. These alterations vary from reduced proliferation of stem and progenitor cells to increased cell death and abnormalities in morphology, connectivity, and localization of young neurons. Although the involvement of the mutual regulation between the HPA axis and the AHN in the pathogenesis of cognitive deficits and mood impairments is evident, several unresolved critical issues are stated. Understanding the details of GC‐mediated mechanisms involved in the alterations in AHN could enable the identification of molecular targets for ameliorating pathology‐induced imbalance in the HPA axis/AHN mutual regulation to conquer cognitive and psychiatric disturbances.
-
Hippocampal hyperglutamatergic signaling matters: Early targeting glutamate neurotransmission as a preventive strategy in Alzheimer's disease
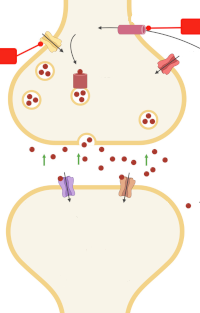
Employees from the Laboratory of Functional Biochemistry of Nervous System highlight a remarkable study in the current issue of the Journal of Neurochemistry in which Hascup and coworkers provide novel data showing that riluzole, an anti‐glutamatergic drug, may be a promising early intervention strategy for Alzheimer's disease (AD), aimed at restoring glutamate neurotransmission prior to amyloid beta (Aβ) plaque accumulation and cognitive decline. The mice APP/PS1, a model of AD, initially are cognitively normal but have elevated glutamate release in the hippocampus at 2–4 months of age. They begin showing cognitive decline and Aβ plaque accumulation at approximately 6–8 months of age, and show obvious AD neuropathology and cognitive impairment at 10–12 months. The riluzole treatment over 4 months (at 2–6 months of age) targeting early changes in glutamatergic neurotransmission prevents cognitive decline observed at 12 months of age and restores glutamatergic neurotransmission. This is one of the most convincing preclinical evidence supporting the idea of targeting glutamate neurotransmission in patients at risk for AD and to use riluzole for this purpose
-
Ca2+-activated KCa3.1 potassium channels contribute to the slow afterhyperpolarization in L5 neocortical pyramidal neurons
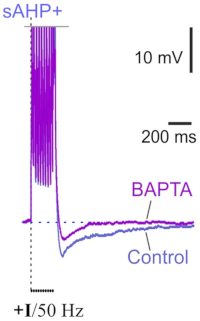
Layer 5 neocortical pyramidal neurons are known to display slow Ca2+-dependent afterhyperpolarization (sAHP) after bursts of spikes, which is similar to the sAHP in CA1 hippocampal cells. However, the mechanisms of sAHP in the neocortex remain poorly understood. Our employee from the Laboratory of Cellular Neurobilogy of Learning identified the Ca2+-gated potassium KCa3.1 channels as contributors to sAHP in ER81-positive neocortical pyramidal neurons. Moreover, their experiments strongly suggest that the relationship between sAHP and KCa3.1 channels in a feedback mechanism underlies the adaptation of the spiking frequency of layer 5 pyramidal neurons. They demonstrated the relationship between KCa3.1 channels and sAHP using several parallel methods: electrophysiology, pharmacology, immunohistochemistry, and photoactivatable probes. Their experiments demonstrated that ER81 immunofluorescence in layer 5 co-localized with KCa3.1 immunofluorescence in the soma. Targeted Ca2+ uncaging confirmed two major features of KCa3.1 channels: preferential somatodendritic localization and Ca2+-driven gating. In addition, both the sAHP and the slow Ca2+-induced hyperpolarizing current were sensitive to TRAM-34, a selective blocker of KCa3.1 channels.