Applied Science
-
Hippocampal hyperglutamatergic signaling matters: Early targeting glutamate neurotransmission as a preventive strategy in Alzheimer's disease
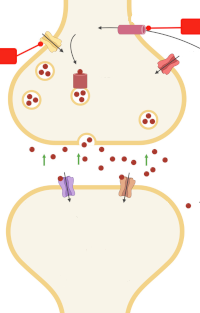
Employees from the Laboratory of Functional Biochemistry of Nervous System highlight a remarkable study in the current issue of the Journal of Neurochemistry in which Hascup and coworkers provide novel data showing that riluzole, an anti‐glutamatergic drug, may be a promising early intervention strategy for Alzheimer's disease (AD), aimed at restoring glutamate neurotransmission prior to amyloid beta (Aβ) plaque accumulation and cognitive decline. The mice APP/PS1, a model of AD, initially are cognitively normal but have elevated glutamate release in the hippocampus at 2–4 months of age. They begin showing cognitive decline and Aβ plaque accumulation at approximately 6–8 months of age, and show obvious AD neuropathology and cognitive impairment at 10–12 months. The riluzole treatment over 4 months (at 2–6 months of age) targeting early changes in glutamatergic neurotransmission prevents cognitive decline observed at 12 months of age and restores glutamatergic neurotransmission. This is one of the most convincing preclinical evidence supporting the idea of targeting glutamate neurotransmission in patients at risk for AD and to use riluzole for this purpose
-
A Translational Study on Acute Traumatic Brain Injury: High Incidence of Epileptiform Activity on Human and Rat Electrocorticograms and Histological Correlates in Rats
In humans, early pathological activity on invasive electrocorticograms (ECoGs) and its putative association with pathomorphology in the early period of traumatic brain injury (TBI) remains obscure. Our scientists from the Laboratory of Functional Biochemistry of Nervous System assessed pathological activity on scalp electroencephalograms (EEGs) and ECoGs in patients with acute TBI, early electrophysiological changes after lateral fluid percussion brain injury (FPI), and electrophysiological correlates of hippocampal damage (microgliosis and neuronal loss), a week after TBI in rats. They revealed that epileptiform activity on ECoGs was evident in 86% of patients during the acute period of TBI, ECoGs being more sensitive to epileptiform and periodic discharges. A “brush-like” ECoG pattern superimposed over rhythmic delta activity and periodic discharge was described for the first time in acute TBI. In rats, FPI increased high-amplitude spike incidence in the neocortex and, most expressed, in the ipsilateral hippocampus, induced hippocampal microgliosis and neuronal loss, ipsilateral dentate gyrus being most vulnerable, a week after TBI. Epileptiform spike incidence correlated with microglial cell density and neuronal loss in the ipsilateral hippocampus. Based on these results, our employees concluded that epileptiform activity is frequent in the acute period of TBI period and is associated with distant hippocampal damage on a microscopic level. This damage is probably involved in late consequences of TBI. The FPI model is suitable for exploring pathogenetic mechanisms of post-traumatic disorders