The new format of online communication that emerged during the pandemic has firmly established itself in our lives, particularly in the fields of education and business. While we have gained more freedom and opportunities through this new medium, many people remain dissatisfied with its remote format. Most people believe that face-to-face communication allows for a better understanding and connection with the other person, as it allows the speaker to feel the energy and emotions of the audience, and vice versa. This is because in addition to verbal information, we also receive a stream of non-verbal cues, such as body language and tone of voice, which help us form a more complete picture of the other person's thoughts and feelings.
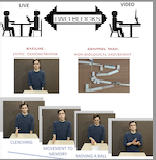
It has been shown that the mirror neuron system and the motor and sensory-motor areas of the cortex play an important role in the perception of nonverbal visual information, including information about the movements, gestures, postures, and facial expressions of others. To investigate how the activity in these visual and sensory-motor regions of the cortex changes during video-based interactions, the authors of article, the researchers of IHNA & NPh RAS analyzed the electroencephalogram (EEG) response of 83 participants who watched identical actions shown live and through video recordings. The experiment was carefully designed to ensure that the images observed by the participants were as similar as possible, whether they were watching live or watching a video recording.
To prevent the spread of the visual alpha-rhythm to the sensory-motor areas of the brain, the independent component analysis (ICA) method was used. This method allows us to separate the mu and alpha components. For the control task, participants were asked to watch a video of a non-biological object, a ball moving through a maze. A static demonstrator was shown as the baseline. Authors analyzed the mu-rhythm response in two frequency ranges: 8-13 Hz and 13-24 Hz. The authors found that the main mu-rhythm, which ranges from 8 to 13 Hz, is highly sensitive to biological and social movements and depends greatly on the format of interaction. Live demonstrations caused a significantly stronger mu-rhythm response in the sensory-motor areas of the brain. The alpha-rhythm, on the other hand, did not respond to the type of biological movement observed. However, live demonstrations initially caused a greater concentration of visual attention, which then decreased to the video format. At the same time, the higher range of the mu-rhythm was more sensitive to various gestures performed by the participants, which should be taken into consideration when developing neuro-interfaces.
Therefore, it is important to understand that remote communication may affect both the concentration of visual attention and the subtle forms of social non-verbal communication related to the motor and sensory-motor areas of the brain.
https://academic.oup.com/cercor/article-abstract/34/4/bhae168/7659152?redirectedFrom=fulltext
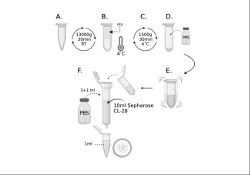
Currently, practically nothing is known about the biological processes occurring in the brain in most mental pathologies. One such pathology is non-suicidal self-injury (NSSI). Researchers from the IHNA&NPh RAS and the Moscow Research and Clinical Center for Neuropsychiatry have outlined a new direction of research - the search for correlates of this condition with the composition of small extracellular vesicles (sEVs) in the blood. The results of a pilot study on this topic were recently published. The work assessed the quantitative characteristics of sEVs in the blood of patients with NSSI and compared the concentration and size of sEVs in patients with major depressive disorder with and without NSSI, as well as assessed the relationship between the sizes and concentrations of sEVs in the sample with such parameters as the severity of anxiety, depression and suicidal risk. The size and concentration of the isolated particles were assessed using dynamic light scattering and nanoparticle tracking analysis.
Authors found that NSSP in people with major depressive disorder is associated with a more severe course of the disorder (greater severity of depression, situational and personal anxiety), as well as a higher risk of suicide. The study did not reveal differences in the quantitative characteristics of sEV in patients with a depressive episode with and without NSSI. The authors believe that future research should be aimed at investigating the structural differences and functional features of sEVs in NSSI.
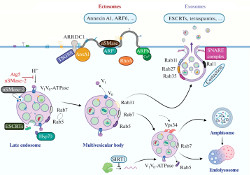
Extracellular vesicles (EVs) are a new and actively developing area of modern experimental and theoretical biology, which attracts researchers primarily by the possibility of using EVs as diagnostic biomarkers and therapeutic agents. Currently, the largest amount of data has been accumulated on small extracellular vesicles (sEVs) - exosomes, vesicles of endosomal origin, and ectosomes (formerly known as microvesicles), which are a product of direct budding from the plasma membrane. In a recent review, authors from the IHNA&NPh RAS and the Moscow Research and Clinical Center for Neuropsychiatry examine the main stages of the biogenesis of exosomes and ectosomes, the main processes of intracellular membrane traffic, as well as signaling with the participation of sEVs. In addition, the authors discuss the role of sEVs in the physiology and pathophysiology of the nervous system, as well as many promising aspects of studying the biology of sEVs.
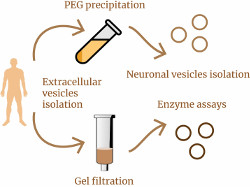
A recently published article by researchers from the IHNA&NPh RAS and the Moscow Research and Clinical Center for Neuropsychiatry raises new and interesting questions in the study of small extracellular vesicles (sEVs) in human blood. Previously, the team, using four different methods, had shown significant increase in the concentration of sEVs in the blood of patients with depression compared to healthy volunteers, and the followed work was intended to clarify the source of these “extra” vesicles in depression. In addition, the authors compared sEVs content in the blood of patients with a wide range of pathologies, including epilepsy, epilepsy with depression, bipolar affective disorder with a current depressive episode, and psychogenic nonepileptic seizures with depression. Small EVs were isolated from patient serum using gel filtration or polyethylene glycol (PEG) precipitation, and both methods showed very similar results. It turned out that in patients with depression, epilepsy, or epilepsy with depression, the total level of mBB in the blood is increased by up to two times compared to healthy volunteers. The authors of the work were able to go further and isolate the fraction of sEVs of neuronal origin (in the blood this is approximately every one hundred and fiftieth vesicle) in these patients, but no difference in the concentration of neuronal sEVs was found between any groups. The question of where the “extra” sEVs in the patients’ blood came from remains open. The authors suggest that the source of these sEVs are immune cells. However, the authors did discover at least one new finding that sheds light on the biogenesis of sEVs. It turned out that sEVs in the serum of both patients and healthy volunteers contain various lysosomal enzymes - and this is a hint that the contents of sEVs may reflect the state of the intracellular endolysosomal system.